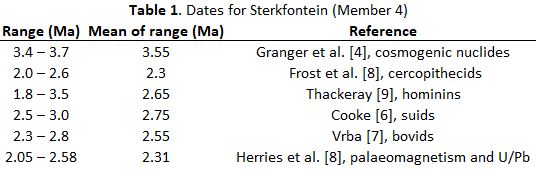
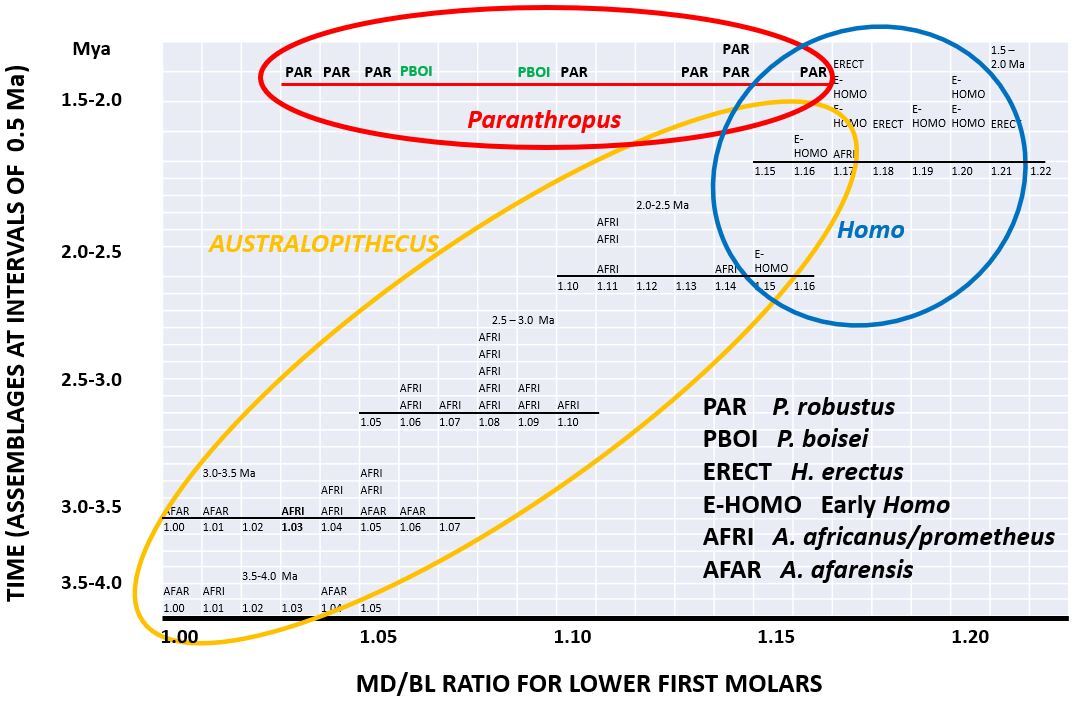
Australopithecus, Paranthropus and Homo are three hominin genera which existed on the African continent during the Plio-Pleistocene. All three genera are represented in Southern Africa (SA) and Eastern Africa (EA), including (among others) A. afarensis (EA), A. africanus (SA) and A. prometheus (SA); P. aethiopicus (EA), P. robustus (SA) and P. boisei (EA); H. habilis (EA), H. rudolfensis (EA), and H. erectus (EA and SA). Phylogenetic relationships between these species are the subject of much debate among palaeoanthropologists, many of whom accept that there are clear boundaries between species, using alpha taxonomy. However, in the context of palaeoecological changes, such boundaries may not always have existed - hence the need for a probabilistic approach such as (for instance) sigma taxonomy. [1,2] In this article I refer to what I call my TERAVEGANT GENEMORPH (TG) model, associated with temperature (TE), rainfall (RA), vegetation (VEG), animals (AN) and hominin species which are the subject of anthropology (ANT), in the context of gene pools (GENE) and morphology (MORPH). It can be expected that in an African Plio-Pleistocene context, dynamic changes in palaeotemperature (TE) would have been associated with Milankovich cycles related to harmonic variability in the earth’s long term motions (precession, obliquity and eccentricity, with periodicities of 20 000, 40 000, and 100 000 years respectively). In terms of the basic TG model, relatively high temperatures (in periods such as the warm Holocene and current Anthropocene) would have been associated with high evaporation off sea surfaces such that (in general) more water would have become available to fall as rain (RA) in terrestrial environments on the African continent. Together these changes in climate (TE and RA) would have been associated with concomitant changes in habitats. For example, under relatively warm and wet conditions in so-called “interglacials”, miombo woodland would have expanded in areas of Zimbabwe, Zambia, and Malawi. By contrast, during relatively cool or cold conditions of TE (so-called “glacials”), rainfall in terrestrial palaeoenvironments would have decreased in general on account of lower evaporation off the sea. As such, miombo woodland (VEG) would have become less extensive, being replaced by savanna grassland species which tolerate relatively low temperatures, such that an ecological “corridor” would have existed between EA and SA (cf. Schrenk et al. [3]). Miombo woodland would have served as a temporary barrier to animals (AN) associated with grassland savanna in EA and SA. There are several such mammalian species in existence today. Wildebeest (Connochaetes taurinus), for example, are grazers which at present have disjunct distributions in EA and SA because of intermediate woodland growing under warm and wet conditions. However, their distributions would have become continuous to some extent during cooler and drier periods when habitats opened up in one or more corridors, allowing for gene flow (GENE) between EA and SA. Like migratory wildebeest (AN), hominins generally associated with savanna vegetation can be expected to have responded intermittently to changes in habitats (VEG) such that gene flow would have occurred between hominin populations (ANT) in Southern and Eastern Africa, when the intermediate miombo woodland fragmented. This holistic (vicariant) scenario is explored here with reference to temporal variability in ratios of hominin molar teeth, as a proxy for morphology (MORPH) as a phenotypic expression of genetic factors (GENE). To place my scenario in a temporal context, based on the TG model, it is first necessary to consider the ages of fossils in EA and SA. Chronology Eastern African fossils representing A. afarensis, H. habilis, H. rudolfensis, and H. erectus can be dated radiometrically using potassium and argon in volcanic deposits. In the absence of active volcanoes in Southern Africa in the Plio-Pleistocene, alternative dating methods are required. For example, Granger et al. [4] have used cosmogenic nuclides (aluminium and beryllium) to date hominins from sites such as Sterkfontein where A. africanus, A. prometheus, and early Homo are represented. Herries et al. [5] have used palaeomagnetic and uranium-lead approaches. Faunal dates have been estimated for Sterkfontein as exemplified by Cooke [6] in relation to suids, by Vrba [7] in relation to bovids, and by Frost et al. [8] in relation to cercopithecids (Table 1). Thackeray [9] described a biochronological method which makes use of the ratio of mesiodistal (MD) and buccolingual (BL) diameters of lower first molar teeth of a diversity of hominin taxa. Using regression analysis, a relationship was obtained between MD/BL ratios and geological ages of well dated Eastern African specimens attributed to Australopithecus or Homo. Knowing MD/BL ratios for Southern African hominin molars representing the same genera, it has been possible to apply the regression equation to estimate ages (Table 1). Temporal changes in tooth ratios MD/BL molar tooth ratios for Eastern and Southern African hominins (Australopithecus, Paranthropus, and Early Homo) are presented in Fig. 1 in which five histograms are shown at intervals of 500 000 years for the period between 1.5 and 4.0 Ma. Dates for Australopithecus from Sterkfontein are those given by Thackeray [9] recognising that the lower estimates indicated by Frost et al. [8] and the upper estimates given by Granger et al. [4] respectively correspond closely to the lower and upper values determined by Thackeray [9]. Conclusion
On the basis of Fig. 1 and the TERAVEGANT GENEMORPH (TG) model, it is hypothesised (firstly) that in the context of palaeoclimatic (TE and RA) and habitat (VEG) changes there were not necessarily clear boundaries between African animal species (AN) and hominin species (ANT) within the period between 3.5 and 1.8 Ma during which hybridisation (GENE) was common (analogous to interbreeding among populations of H. sapiens, H. neandertalensis, and Denisovans in the late Quaternary), contributing to variability in anatomical morphology (MORPH); that (secondly) an African gene pool consisting of interacting A. africanus, A. prometheus, and A. afarensis populations existed between 3.0 and 3.5 Ma among these three taxa, having no distinct boundaries in the context of gene flow and episodic changes in habitats in Eastern and Southern Africa, representing a common ancestral group for Paranthropus and Early Homo; that (thirdly) at the level of a genus there was not a clear boundary between Australopithecus and Homo; that (fourthly), at the level of a species the transition between A. africanus and Homo habilis constituted a chronospecies 10 (an anagenetic transition between species without clear boundaries) between at least 2.5 and 1.8 Ma; and (fifthly) that there was no clear boundary between P. robustus and P. boisei in the context of episodic changes in habitats in Eastern and Southern Africa. Acknowledgements I thank Marine Cazenave, Peter Knox-Shaw, the late Sue Dykes and anonymous commentators. References 1. Thackeray JF. 2018. Alpha and sigma taxonomy of Pan (chimpanzees) and Plio-Pleistocene hominin species. South African Journal of Science, 114:1-2. 2. Thackeray, JF. and Schrein, CM. 2017. A probabilistic definition of a species, fuzzy boundaries and ‘sigma taxonomy’. South African Journal of Science 113(5/6): 1-2. 3. Schrenk, F., Kullmer, O., Sandrock, O. et al. 2002. Early Hominid diversity, age and biogeography of the Malawi-Rift. Human Evolution, 17: 113–122. 4 Granger, DE., Stratford, D., Bruxelles, L., Gibbon, RJ., Clarke, RJ. and Kuman, K. 2022. Cosmogenic nuclide dating of Australopithecus at Sterkfontein, South Africa. Proceedings of the National Academy of Science USA, 119:27. 5. Herries, AIR., Pickering R., Adams JW., Curnoe, D., Warr, G., Latham, AG. and Shaw J. 2013. A Multi-Disciplinary Perspective on the Age of Australopithecus in Southern Africa. In The Paleobiology of Australopithecus. Editors Kate E Reed, John G Fleagle, Richard E Leakey. Dordrecht: Springer, 21–40. 6. Cooke, HBS. 1974. Plio-Pleistocene deposits and mammalian faunas of Eastern and Southern Africa. In Proc. Ve Congrès Neogène Mediterranéen, Lyon. Mémoir 78(1) 1971. Paris: BRGM. 99-108; 1974. 7. Vrba, ES. 1982. Biostratigraphy and chronology based particularly on Bovidae of southern hominid-associated assemblages. Proc. 1st Intl. Cong. Human Palaeont., Nice. Paris: CNRS. 2:707-752. 8. Frost, SR., White, FJ., Reda, HG. and Gilbert, CC. 2022. Biochronology of South African hominin-bearing sites: A reassessment using cercopithecid primates. Proceedings of the National Academy of Science USA, 119:45. 9. Thackeray, JF. 2023. A tribute to Yves Coppens: age estimation of Australopithecines in South Africa. Bulletin du Musée 39;anthropologie préhistorique de Monaco (Bulletin of the Museum of prehistoric anthropology of Monaco); Hors-série 10:23-27. 10. Thackeray, JF. 2015. Homo habilis and Australopithecus africanus, in the context of a chronospecies and climatic change. In: Runge, R., (ed.) Changing climates, ecosystems and environments within arid southern Africa and adjoining regions. Palaeoecology of Africa, 33:53-58. 11. Thackeray, F. and Dykes, S. 2023. Biochronological ages for South African Australopithecus and a Plio-Pleistocene African hominin lineage (1,5 – 3,5 Ma)? The Digging Stick, 40 (1):11-12.
0 Comments
Leave a Reply. |
Palaeontological Society of Southern AfricaPalaeoShwe art by Sciism & Puppet Planet © 2022
|
Contact us here: |